Cookie preferences
Choose which categories you allow. Necessary cookies are always on.
Plasma 101
Who: Eligible donors between 18 and 64 can earn up to $560 a month in NY and up to $770 a month in FL.
What: Plasma is the yellow part of your blood that replenishes naturally.
Where: Queens, Brooklyn, The Bronx (NY), and Ft. Pierce (FL).
Why: Get paid to donate and help treat bleeding disorders, immune deficiencies, and more.
When: No appointment needed—walk in anytime before closing.
The Fascinating Journey of Plasma from Donor to Recipient
The Fascinating Journey of Plasma from Donor to Recipient
Saving and improving patients’ lives begins with the selfless gift of plasma donation. Yet few appreciate the intricate journey this precious biologic undertakes, traversing intricate supply chains with demanding oversight to arrive safely to those whose survival depends on it. By illuminating the meticulous steps and stakeholders participating behind the scenes, this piece hopes to inspire deeper admiration for an intricate system delivering healing far and wide.
Plasma takes a 45-90 minute journey from altruistic donors giving 650-825mL to collection centers for extensive safety testing. It then ships in temperature-controlled containers across thousands of miles to hospitals where technicians match it to recipients. Finally, nurses infuse the lifesaving plasma into patients at 1-2mL/kg/hour while monitoring for rare reactions.
Journey and Processing of Plasma:
Step | Description |
Plasma Composition | Clear, straw-colored liquid; over half of blood volume; contains proteins, nutrients, electrolytes, clotting factors. |
Key Plasma Proteins | Albumin (maintains pressure, transports substances), Globulins (immune function, transport), Fibrinogen (blood clotting). |
Plasma Origins | Liquid component of blood, circulates in the vascular system, distributes nutrients and waste. |
Testing and Processing | Extensive safety testing, pathogen reduction methods (NAT assays, solvent detergent treatment, nanofiltration, methylene blue dye). |
Components and Derivatives | Cold ethanol fractionation for albumin, immunoglobulins; used in therapies for hemophilia, immune deficiencies. |
Inventory Specifications | Frozen plasma is typically stored at or below −18°C for up to one year; stringent storage protocols. |
Distribution Logistics | Complex supply chain with demand planning, transport protocols, temperature-controlled shipping. |
Hospital Workflows | Blood bank operations, crossmatching, patient infusion protocols for safe administration. |
The Composition of Plasma
Plasma is a clear, straw-colored liquid that accounts for over half of the total blood volume. It is comprised of various proteins, nutrients, electrolytes, clotting factors, and other organic substances suspended in fluid. These components work together to regulate essential bodily functions and transport vital resources throughout the vascular system.
Key Contents of Plasma
The major proteins found in plasma include albumin, globulins, and fibrinogen. Albumin helps maintain colloidal osmotic pressure to prevent fluid from leaking out of blood vessels. It also binds to hormones, neurotransmitters, medications, and various metabolites and transports them throughout the body.
Globulins have wide-ranging responsibilities from transporting iron, lipids, and vitamins to bolstering immune function. One type of globulin, called immunoglobulins, recognizes foreign substances like bacteria and viruses, facilitating their destruction.
Fibrinogen, along with other clotting factors like prothrombin, enables blood clotting whenever vessels are damaged. Upon injury, fibrinogen converts to fibrin threads, forming mesh-like clots to prevent further blood loss.
Besides proteins, plasma contains electrolytes like sodium, potassium, chloride, bicarbonate, and calcium that regulate fluid balance, nerve conduction, muscle function, blood pH, and bone strength. Organic metabolites found in plasma include glucose, lipids, amino acids, and urea, which provide energy, synthesize important compounds, and transport waste.
The Origins of Plasma
Plasma originates as the liquid component of blood, where it suspends the cellular elements—red blood cells, white blood cells, and platelets. Comprising 55% of total blood volume, this straw-colored fluid circulates throughout the 60,000 miles of blood vessels, capillaries, venules, and arterioles that vascularize the body. Plasma flows through these vessels, distributing vital nutrients, clotting elements, immunological factors, hormones, neurotransmitters, and metabolic waste products to and from tissues.
Capillaries provide the interface where small molecules like water, ions, gases, glucose, urea, and amino acids diffuse between plasma and the interstitial fluid surrounding cells. Larger molecules rely on specialized transport mechanisms to cross capillary barriers.
Applications of Plasma Components
Many plasma constituents, like clotting factors, immunoglobulins, and albumin, now have therapeutic applications for various diseases. Clotting factor concentrates treat bleeding disorders like hemophilia, where patients lack these coagulants.
Prothrombin complex concentrates containing vitamin K-dependent factors are administered during emergencies like trauma or surgery. For patients unable to produce immunoglobulins, plasma derivatives like intravenous immunoglobulin (IVIG) or Rho(D) immune globulin (WinRho) bolster defenses against infection.
Albumin solutions help restore blood volume after extensive burns, trauma, or surgery-related blood loss. Clearly, the individual components making up this vital bodily fluid each serve indispensable roles in maintaining homeostasis.
Clinical Applications and Donor Process:
Aspect | Description |
Therapeutic Applications | Clotting factors for hemophilia, immunoglobulins for immune deficiencies, albumin for blood volume restoration. |
Donor Eligibility | Health screening, weight and medical history assessments, deferrals for certain conditions and medications. |
Donation Process | Apheresis collection, post-donation care, compensation models. |
Safety Measures | Pathogen testing, donor health protection, preventing transmission of infectious diseases. |
Post-Donation Care | Bandage care, activity restrictions, hydration for maintaining health and wellness, and protein replenishment. |
Plasma Testing | Nucleic acid testing for viruses, pathogen reduction steps, quality assurance. |
Inventory Management | Forecasting models, allocation algorithms, transportation logistics, monitoring systems. |
Clinical Administration | Physician ordering, blood bank operations, patient infusion protocols, reaction management - preventing and treating shock. |
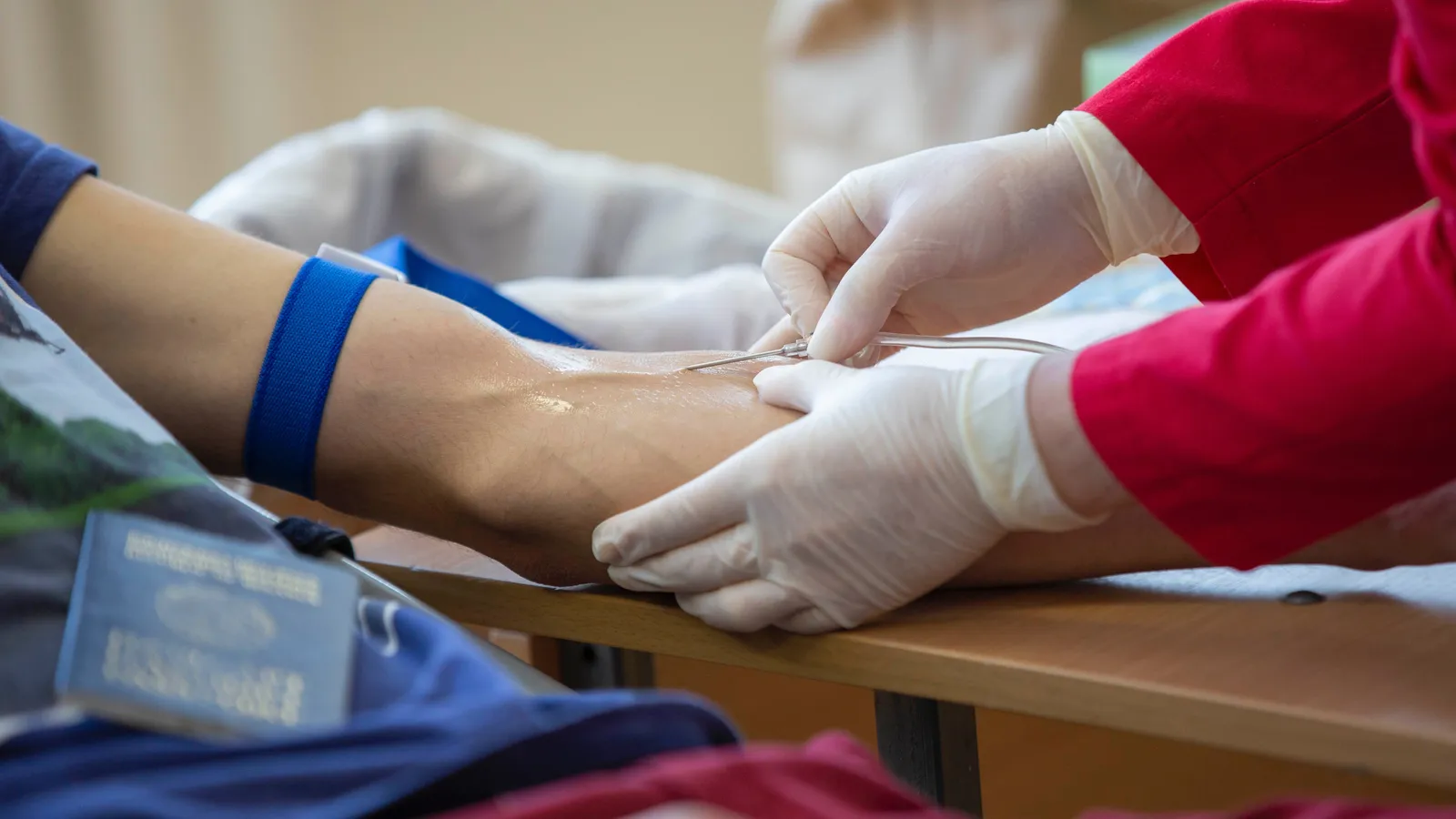
Becoming a Plasma Donor
How does donating plasma work? Donating lifesaving plasma starts with determining if you meet the eligibility criteria. Candidates must undergo screening and assessments to ensure plasma can be collected safely without posing undue risks. Once qualified, donors visit plasma collection centers where automated apheresis machinery withdraws plasma while returning red blood cells and platelets to circulation. Afterwards, donors learn about post-donation care instructions and compensation models that provide financial incentives to return frequently.
Donor Qualifications and Lifestyle Factors
Prospective donors complete health histories detailing any medical conditions, medications, recent tattoos/piercings, travel locations, or high-risk behaviors that could compromise donation safety or plasma quality.
Certain factors like viral infections, underlying chronic illness, weighing under 110 pounds, or recent childbirth may warrant temporary deferral, but not necessarily permanent restrictions. Medications like blood thinners also require temporary deferral until an appropriate washout period has passed. However, conditions causing low protein or hematocrit levels may exclude plasma donation.
These stringent requirements exist firstly to protect donor health and safety. Secondly, they prevent the transmission of infectious pathogens that could harm fragile recipients. By self-deferring for any concerning medical issues or recent exposures, donors help ensure plasma retains purity for final derivative products.
The Plasma Donation Process
Qualified donors first sanitize their arms before a trained phlebotomist inserts a sterile needle to gain intravenous access, usually in the crook of the elbow. Donors recline in a padded chair while a technician connects them to an automated apheresis device, priming the tubing with sterile saline and anticoagulant citrate to prevent clotting.
During the roughly 45-90 minute process, blood flows from the arm into a centrifuge, separating plasma from cellular components. A portion (650-825mL) of plasma is retained while red blood cells and platelets continuously return to circulation in saline solution. Technicians monitor vital signs, blood flow, plasma volumes collected, and watch for adverse donor side effects, like dizziness or numbness from citrate. Total blood outside the body at any moment is less than one cup.
After the Donation
When target plasma volumes are obtained, technicians clamp tubing and withdraw needles, applying a bandage and cold compress to prevent bruising and irritation. Donors then rest for around 15 minutes with light refreshments and a salty snack, helping replace fluids and proteins lost during donation.
Donors learn proper bandage care and restrictions on rigorous exercise for the remainder of the day to avoid reopening the needle site. Normal activities can be resumed the next day, but strenuous exercise should wait 48 hours post-donation.
Donors are informed on the earliest date eligible to return based on gender, weight, and total plasma volumes donated, while still allowing healthy replenishment between visits. Compensation models include base donation fees plus incentives like drawing cash prizes for referrals or achieving bonus visit milestones.
By providing flexible scheduling around work/life demands plus financial compensation modeling altruistic tendencies, Olgam Life aims to make the donation process intrinsically as well as extrinsically rewarding to inspire sustained donor dedication.
Testing and Processing Plasma
Looking from donation to medical use, plasma undergoes extensive safety testing and inventory tracking from collection agencies to final pharmaceutical manufacturers. Robust procedures inactivate pathogens, fractionate components, and ensure quality preservation along each supply chain step.
Pathogen Reduction Methods
Prior to release, donated plasma is tested via nucleic acid testing (NAT) assays for dangerous viruses like HIV, hepatitis B/C, West Nile, Zika, and others. Any reactive units get discarded, while non-reactive plasma pools still undergo additional “multilayered” pathogen reduction steps, destroying or removing agents that tests may have missed.
To ensure safety, manufacturers utilize solvent detergent treatment, incubating plasma in the organic chemicals tri-n-butyl phosphate and Triton X-100, which dissociates viral envelopes. Similarly, nanofiltration uses 15-20nm pore filters, trapping viruses by size exclusion.
Lastly, adding methylene blue dye then illuminating plasma with visible light triggers free radical reactions, destroying viral RNA and DNA through oxidative damage, preventing replication. Combined, these multilayered virus attenuation steps minimize any possible pathogen transmission.
Components and Derivatives
After pathogen reduction, plasma undergoes cold ethanol fractionation to derive protein components like albumin and immunoglobulins or precipitate out cryoprecipitate containing fibrinogen and factor VIII for hemophilia therapy.
Other key derivatives include prothrombin complex concentrates with vitamin K-dependent clotting factors and Rho(D) immune globulin given during pregnancy to prevent hemolytic disease from Rh blood type incompatibility. Plasma proteins get purified, formulated, filled into vials, then stored frozen, awaiting distribution to healthcare settings.
Inventory Specifications
Both plasma and resultant protein fractions followa stringent storage protocol,s preserving integrity. Frozen plasma maintains potency specifications for 3 years, while thawed plasma gets just 1 year of shelf-life. Optimum preservation occurs by continuously storing inventory at -18 degrees Celsius, avoiding repeat freeze-thaw degradation. Similarly, albumin and clotting factor concentrates often persist frozen for years.
Throughout cold chain distribution, oversight bodies also track plasma units to ensure appropriate release and legitimate use. Applying ISBT 128 information standard barcodes on containers allows monitoring custody changes among collectors, testers, fractionators, distributors, and hospital clients accessing detailed audit trails. Such oversight reduces possibilities products get illegally diverted or misused.
By upholding the highest testing, processing, and tracking standards, players across the plasma supply continuum collectively ensure the safety and efficacy of this precious donated gift, benefiting fragile recipients.

Distributing Plasma Inventory
Delivering plasma from donors to patients spanning thousands of miles while preserving safety and efficacy poses complex logistical coordination. Sophisticated supply chain practices encompassing demand planning, transport protocols, and tracking systems ensure this critical biologic resource remains stabilized and secured in transit across vast geographies.
Ordering and Allocation Algorithms
To align supply with demand, planning teams utilize historical usage, clinical developments, and population data developing robust demand forecasts assisting allocation decisions. Automated reordering also helps maintain 2-4 weeks of inventory buffer responding to fluctuating needs.
When scarce products like AB plasma become limited, priority matrices deliver inventory to trauma centers, burn wards, and neonatal ICUs first over less urgent requests. Proximity-based distribution models also minimize transit times by routing products to proximate hospitals as contingencies allow rather than distant ones.
This centralized planning and risk-adjusted allocation by regional distribution hubs helps maximize inventory utilization despite unpredictability.
Transportation Logistics
Where does your plasma go once you’ve donated? Whether shipping short distances ground or overseas air cargo, strict FDA regulations safeguard plasma integrity in transit. Approved containers and qualified refrigerants keep ambient temperatures 1–10°C throughout travels bypassing temporary excursions up to 40°C. Passive methods using cold packs suffice brief regional deliveries while active refrigeration proves essential for longer duration air freight.
Shipments carry data loggers continuously capturing ambient conditions and GPS location in real-time. Any temperature, route, or hold-time deviations trigger alerts to intercept compromised goods.
Barcode tracking of serial numbers via scanning also traces chain of custody through each distribution handoff. Such oversight reduces tampering, expiry, or illegitimate use risks despite the complexities transporting biologics globally.
Inventory Monitoring
Beyond transit, computerized systems tracking plasma location and ownership changes provide end-to-end supply chain transparency on collected units source to final disposition per FDA mandates. Warehouse management systems generating inventory reports also assist planning appropriate buffers mitigating stock-outs or losses from expiry.
Applying integrated ISBT 128 information standard barcodes on containers allows reliably identifying and tracing inventory distribution events through each system accessing custody including collection agency, testing lab, distributor, healthcare facility, and any intermediaries in between. Such oversight access facilitates interventions whenever process disruptions, adverse weather, or other logistical barriers threaten delivery timeliness.
By upholding meticulous monitoring and control standards across fragmented distribution channels, plasma integrity and availability persists benefiting vulnerable end recipients.
Hospital Plasma Workflows
Within healthcare facilities, robust workflows ensure physician-ordered plasma gets administered safely to intended patients. Order entry protocols, blood bank crossmatching procedures, and bedside positive identification all collectively uphold the chain of assurance protecting recipients.
Physician Ordering Process
When clinician assessment determines plasma could benefit patients exhibiting coagulation deficiencies from liver failure, trauma, or surgery complications, physician orders get entered into the electronic medical record detailing the indication, dosage, duration and delivery specifics.
Computerized provider order entry applies clinical decision support references like dosing calculators and diagnosis-linked components selecting most appropriate agents whether fresh frozen plasma, cryoprecipitate, or factor concentrates.
After transmitting orders to blood bank for fulfillment, documentation ties the resulting infusion back to the original order through barcoded specimen labeling and scanned product verification upstream and at bedside downstream.
Blood Bank Operations
Given ABO compatibility drives plasma suitability, blood bank technicians first type patients to identify ABO/Rh status while also testing for irregular antibodies that could provoke immune reactions. They then run crossmatch tests combining patient sera with potential units to highlight incompatibilities obstacles needing resolution before release.
Accessing inventory management systems indicates logistical limitations like blood groups lacking available stock or short-dated collections nearing expiry possibly requiring ordering alternative products from elsewhere. Once identifying and reserving appropriately matched units not otherwise committed, labeling and transporting those to procedures/bedside proceeds contingent on final, confirmatory checks upholding safe administration.
Patient Infusion Protocols
Prior to infusion, nurses confirm patient identity via wristband scans matching workstation printed labels. They examine transfusion orders and product labels to verify concordance of recipient/unit details. Finally, bedside barcode scanning the linked patient-unit pair must aligned before spiking bags to initiate infusion pumps.
Vital signs get monitored over first fifteen minutes watching for adverse reactions before continuing the hour-long transfusion. Nurses check for infiltration at the IV site or other complications throughout while recording volumes infused, patient tolerance and any issues emerging.
Such rigorous validation, monitoring and documentation protocols across the patient plasma continuum maintain safe and appropriate administration protecting vulnerable recipients through delivery completion.
Infusing Plasma into Patients
Administering plasma requires meticulous setup validating correct patient, product, dosing, rate and route to uphold safe delivery. Continuous monitoring thereafter watches for subtle signs of evolving reactions needing rapid intervention to stabilize affected patients.
Infusion Setup and Initiation Protocols
After double nurse confirmation of patient identity, orders, and matched unit, infusion pumps are assembled correctly, programming drug name, patient identifiers, rate, and volume specifications. Primary tubing sets get spiked into bag ports priming lines to evacuate air before connecting central or peripheral catheters avoiding introducing new bubbles.
Once linked to patient access, pump cassettes load, volume to be infused keys in and rates set between 1-2 mL/kg/hour fall within safe range. Tubing tappers eliminate trapped bubbles before starting infusion verifying smooth flow through drip chambers without infiltration around catheter sites. Initial 5-10 minutes run slowly observing for acute reactions prior to advancing toward target dose.
Transfusion Monitoring Requirements
Baseline vital signs like temperatures, pulse, respirations and blood pressure establish pre-transfusion norms for comparison once infusion initiates. Monitoring repetition occurs every 5 or 10 minutes thereafter assessing subjective tolerance and objective changes.
Nurses visually inspect for new rashes, flushing or swelling suggesting escalating reactions. They palpate for new onset fever and utilize pulse oximetry continuously tracking circulating oxygen levels. Intake balances against volume infused subtracts outputs quantifying absorption. This meticulous charting captures early trends potentially indicative of evolving complications.
Identifying and Managing Reactions
Rapid rises in temperature, erythema rashes, dyspnea and falling blood pressure should trigger immediate pump suspension and calling rapid response teams to bedside. Lab work like histamine levels, chest films to evaluate pulmonary fluid overload, and culprit unit retention for technician evaluation all expedite reaction classification.
Mild nuisance reactions like transient flushing or pruritis may continue with supportive diphenhydramine or acetaminophen administration alleviating symptoms. However, severe manifestations like anaphylactic or TRALI reactions necessitate urgent interventions like epinephrine, steroids, diuretics and supplemental oxygen administration in ICU settings to stabilize hypotension, pulmonary edema and shock until crises resolve.
By recognizing the earliest subtle signs of reactions through meticulous monitoring, nurses play pivotal roles minimizing morbidity through urgent alert triggering enabling providers rapidly apply appropriate countermeasures decompressing consequences before escalating life threats evolve. Their vigilance truly upholds patient protection throughout.

Optimizing Plasma Inventory Management
Balancing plasma supply with unpredictable demand requires proactive planning tactics and emerging technologies expanding donor pools while ensuring optimal utilization efficiency guiding judicious administration.
Inventory Forecasting Models
By data mining historical electronic health record transactions like prior plasma orders and component usage logs, demand forecasting analytics apply machine learning algorithms predicting likely monthly utilization rates. This facilitates adjusting reorder points and on-hand buffers to meet probable needs amid fluctuating trauma admissions and elective surgery cycles.
Crossmatching algorithms also model regional requirements consolidating spare O negative units inventories into central depots readily accessible for mass casualty events. Reallocating excess collections based on perishability risks rather than just facility ownership helps maximize availability systemwide protecting all hospitals potentially needing emergency plasma.
Expanding Plasma Collection
With trauma needs growing, creative approaches to expand donor pools help boost reliable inventory. Targeted digital marketing guided by behavioral science insights resonates with potential new donors appealing to motivations like community service. High school blood drives foster first-time teenage donors eventually becoming lifetime regulars.
Olgam Life bridges convenience gaps leveraging agile neighborhood plasma collection centers with flexible evening hours located along bus routes retaining loyal, committed donors through customer-centric care. Recent approval allowing small precise skin pricks facilitating mobile phlebotomy opens additional site options conveniently capturing new candidate groups.
Compensation models including bonus structures after every fifth donation further incentivize frequent return visits. Such creative avenues collectively expand donor pools securing consistent inventory buffers protecting stock-outs when sudden utilization surges emerge.
Future Directions
Ongoing advances around plasma concentrate both collection optimization and judicious utilization promoting positive outcomes. Pathogen reduction technology like methylene blue photodynamic treatment now undergoing clinical trials neutralizes infectious threats during manufacturing. Freeze-dried plasma products currently in testing store safely at room temperature ideal for military settings lacking cold chain logistics.
Patient blood management programs optimize red cell levels before major elective surgeries through IV iron and erythropoietin, reducing substantive plasma needs intraoperatively. Real-world evidence assessing correlations between plasma volumes transfused and mortality rates informs data-driven thresholds ensuring only appropriate patients focusing on shock, coagulopathy and massive transfusion receive plasma balancing efficacy with safety.
By embracing technology securing optimal collections from expanded donor pools while high-quality data guides efficient allocations and responsible stewardship, the reliability, safety and judicious utilization of this extraordinary gift of life all continue advancing benefiting recipients through coming decades. Get in touch and chat with us if you have any questions!